Our Story
Engineering CPR was established in 2013 when Taras Worona, decided to strike out on his own. With 30 years of product development experience under his belt, in both large and small companies, Taras was confident he could help propel products and companies to success. He started drawing on his network of senior designers and specialists and assembled a team. He began putting the Engineering CPR name out, and soon enough we were able to start
providing various clients with accelerated solutions in design, prototyping, testing, and certification management.
One notable early engagement was to redesign a hospital room sterilization system. The team had a prototype but was struggling with the last couple steps before they could commercialize the product. Luckily, a past colleague of Taras’ was able to bring Engineering CPR in to help. Engineering CPR improved the product for manufacturability and usability; and made changes enabling it to meet 3rd party safety testing to the IEC 61010 and EMC standards.
Our most foundational engagement was 7D Surgical (now Seaspine); a then small company that was developing a Surgical Navigation System. Engineering CPR was first only tasked with their electrical cabling design, but with that success, eventually took over the mechanical design from Celestica as well as the management of the mechanical design of the cart by a third party.
Engineering CPR finished the design that Celestica started and managed the completion of other aspects of the mechanical and electrical design.
To help 7D Surgical move into production, Engineering CPR opened a facility in Mississauga, Canada. There, the initial prototypes of the 7D Surgical design were manufactured and our Quality Management System was purpose built to be ready for medical device production.
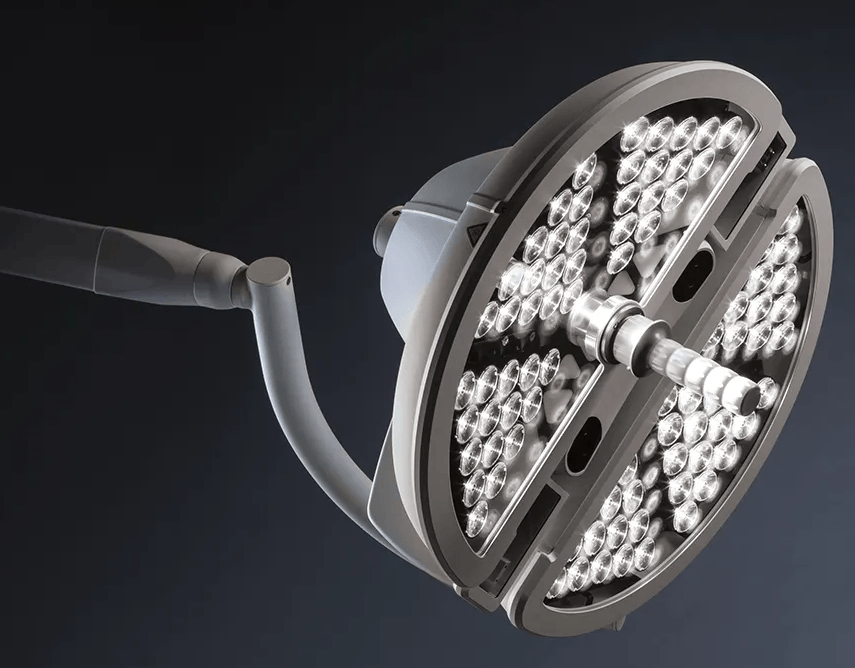
The design and prototypes built were adequate to enable 7D Surgical to complete 510k testing and 3rd party safety & EMC testing. During the waiting period for FDA approval, Engineering CPR embarked on an industrial and manufacturability redesign. Engineering CPR was able to redesign the 7D Surgical head to make it both look impressive and be easy to manufacture; resulting in the design that is sold today.
We continue to manufacture the 7D Surgical System to this day.
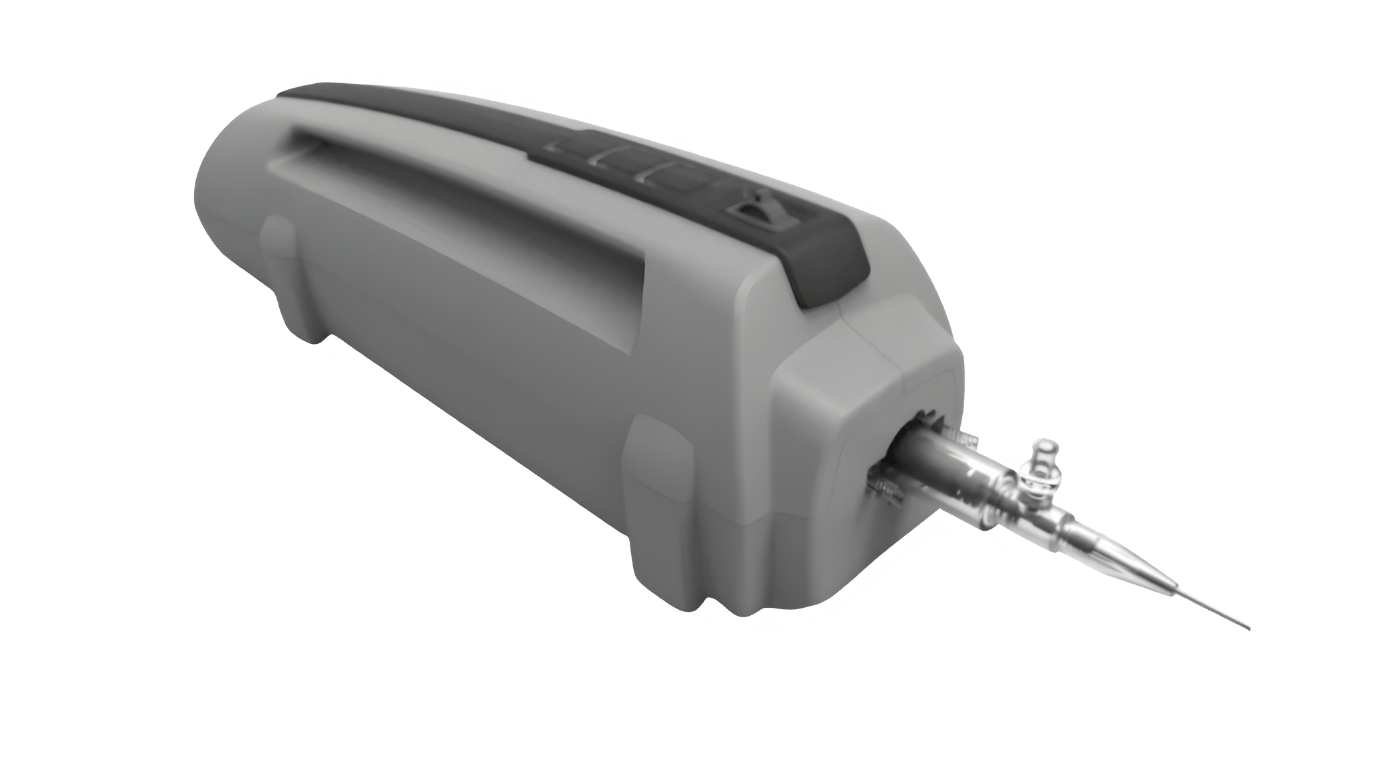
At the referral of 7D Surgical from their positive experience with us, Conavi Medical engaged Engineering CPR to design their mechanical and electrical catheter actuation system and interface. The scope of our work was the plastic part design for the disposable catheter, PCBA design, mechanical assembly, and motor integration. The device functions include locking a catheter in place, mating to a rotating part of it and spinning at a high rate to enable cardiac imaging. Engineering CPR was able to develop and produce the system that was used in the 510k and 3rd party certification testing.
Since these early engagements we have worked on projects in the
medical, industrial and consumer industries
for both design and manufacture, some of which can be seen
here.
In 2019, we moved to a larger facility beside Toronto Pearson Airport to help us proactively meet the growing needs of our client base and to give us an easy access to worldwide distribution channels.
Over the years we have employed lean methodologies, to focus our activities on work that is valuable to our customers. We employ a 6S System (Safety, Sort, Set-in-order, Shine, Standardize, Sustain) to help maintain streamlined manufacturing services.
We have developed our time-tested React™ hybrid-QMS to help us measure where our quality stands and where it is headed so that we can and proactively improve our processes.
We have developed our modular Reliance Program to provide clients with tailored material compliance solutions for declaring RoHS 3, REACH, SCIP, Prop 65, Conflict Minerals compliance.
We aspire to not only grow, but to become the industry leaders in design and manufacturing best practices. To do that, we employ a cultural willingness to try new things, to measure both our successes and failures and to move forward with a deeper understanding of what works and what does not.
If you have similar lofty aspirations for your company and want a committed partner that you can rely on –
we invite you to reach out.